




Heel pain?
These heel pads are designed to raise your heel. They are indicated in cases of Achilles tendonitis, talalgia or heel spur. These silicone heel pads provide cushioning to reduce shock.
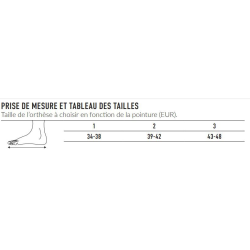
For conditions that can benefit from heel elevation, including the following:
Support zone for improved heel placement
Three removable layers, each 9 mm high
Easy to use in a high-top dress shoe
Specific References
A historic French brand, Gibaud weaves a unique story in its Saint-Etienne and Trévoux workshops: that of developing textile solutions dedicated to the health of the whole family, to prevent, preserve and relieve at every stage of life.
Specializing in back, wrist, ankle and knee care (orthopedics), leg care (phlebology) and foot care (podology), Gibaud cares for people throughout their lives, facilitating their mobility, and contributing to their well-being by acting on the body and mind of those who wear the care.
For conditions that can benefit from heel elevation, including the following:
Post-surgical Achilles tendon rehabilitation
Achilles tendon rehabilitation following non-surgical treatment.
No known contraindications.
Precautions :
Under medical supervision, gradually remove the different levels of thickness, starting with the lower level in contact with the shoe (the darkest color).
The lightest part of the heel lift should be removed last (after symptoms have disappeared).
Do not wear for prolonged periods without medical supervision.
The brace must not be placed in direct contact with injured skin.
This orthosis is a single-patient device. Do not reuse on multiple patients.
If any problems arise during use of this orthosis, such as pain or the appearance of local signs, remove the device and contact your healthcare professional.
If any serious incident occurs in connection with the device, the healthcare professional and/or patient should report it to the manufacturer and the competent authority in the country concerned.
